|
JINAN, China, Dec. 25, 2024 /PRNewswire/ -- During the 66th Annual Meeting of the American Society of Hematology (ASH) held in San Diego from December 7 to 10, preliminary results from the first human trial of QLS32015, a novel anticancer drug developed by Qilu Pharmaceutical for treating relapsed/refractory multiple myeloma (RRMM), were unveiled (Poster Number: 1990). The findings demonstrated that QLS32015 showed excellent anti-tumor activity and was well tolerated by patients with RRMM.
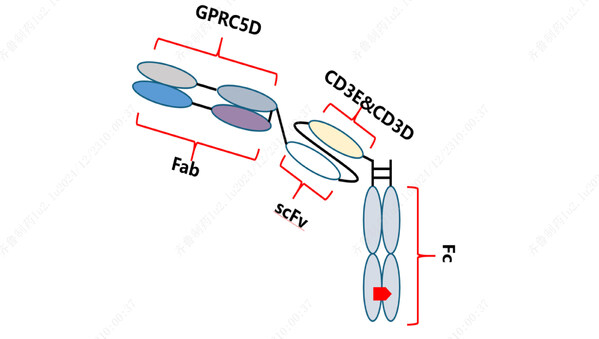
QLS32015, a novel humanized IgG1 T-cell retargeting bispecific antibody, targets both GPRC5D, a G protein-coupled receptor class C group 5 member D, and CD3. By bridging CD3-expressing T cells and GPRC5D-expressing tumor cells, QLS32015 facilitates T-cell-mediated destruction of cancer cells. Instead of relying on the conventional major histocompatibility complex (MHC) and specific T-cell receptor (TCR) binding pathways, this process creates an immune synapse that activates T cells, triggering them to attack and kill tumor cells. [1-2]
This study is an open-label, dose-escalation/expansion Phase I clinical trial (NCT05920876), aiming to assess the safety of QLS32015 in patients with RRMM while offering preliminary insights into its efficacy. The study is led by Prof. Lugui Qiu from the Institute of Hematology and Blood Diseases Hospital at the Chinese Academy of Medical Sciences.
The study enrolled patients with RRMM who had either progressed on or shown intolerance to existing treatments. These patients received QLS32015 as monotherapy, administered subcutaneously at doses ranging from 2 to 200 μg/kg, either weekly or biweekly. During the Phase Ia dose-escalation phase, the primary endpoints were to identify the dose-limiting toxicities (DLT), the maximum tolerated dose (MTD), and the recommended phase 2 dose (RP2D). The study design employed a combination of accelerated titration and Bayesian optimal interval strategies.
As of August 31, 2024, a total of 13 patients had been enrolled in the trial. The median age among these participants was 61 years, and they had undergone a median of 3 prior lines of therapy (range, 1 to 8). Notably, 76.9% of the patients had received at least triple therapy, which included proteasome inhibitors, immunomodulators, and anti-CD38 monoclonal antibodies. Additionally, 23.1% of the participants had been treated with B-cell maturation antigen (BCMA) chimeric antigen receptor T-cell (CAR-T) therapy.
In terms of safety, one DLT was observed at the 54 μg/kg dose level, but the MTD was not reached. Treatment-related adverse events (TRAEs) were reported in all patients. The most common TRAE was cytokine release syndrome (CRS), occurring at grades 1 or 2 in all patients, with a median duration of 2.1 days. The most common grade ≥3 TRAEs were hematological toxicities, including lymphopenia (92.3%) and thrombocytopenia (61.5%). Most non-hematological TRAEs were grade 1 or 2. Importantly, no cases of immune effector cell-associated neurotoxicity syndrome (ICANS) were reported, and there were no TRAEs that led to treatment discontinuation or death.
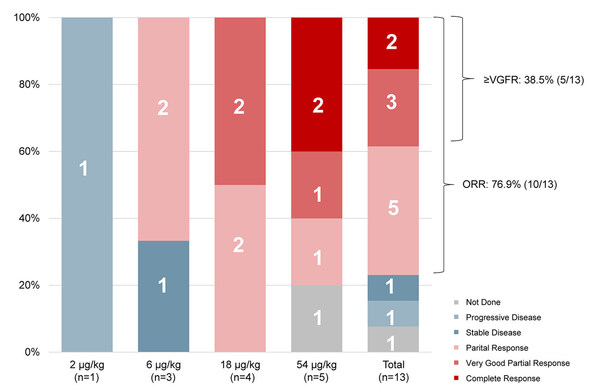
As of the data cut-off, 12 out of the 13 enrolled patients had undergone at least one assessment of efficacy. The objective response rate (ORR), assessed according to the International Myeloma Working Group (IMWG) criteria, was 76.9% (10/13). Among these patients, two (15.4%) achieved complete response (CR), three (23.1%) reached very good partial response (VGPR), and five (38.5%) attained partial response (PR).
The findings suggest that QLS32015 has a tolerable safety profile and shows promising remission rates among RRMM patients. The dose-escalation study is still in progress. DLT, MTD, and RP2D are to be determined.
References: |
1. Li J, et al. 2017;31(3):383-395. |
2. Velasquez MP, et al. Blood. 2018;131(1):30-38. |